- Solid-closely packed in an orderly manner-strong bonds, vibrates at a fixed position.
- Heat energy converts to kinetic energy (K.E.) K.E. goes up, vibrate a little faster, temperature goes higher.
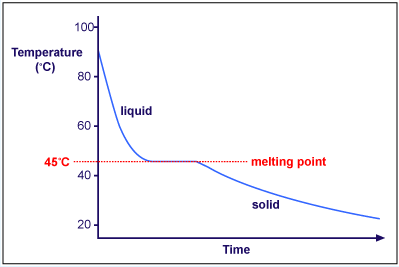
- Solid to liquid-temperature remains constant because heat is used to overcome forces of attracton that hold the particles together-melting point-heat released is called latent heat of fusion.
- Liquid (All solid has melted)
- Temperature goes higher, particles move faster, K.E. up.
- Some molecules start to move freely. Starts changing from liquid to gas.
- Liquid to gas.Temperature stays same. Heat is used to overcome the forces of attraction that hold the particles together. Boiling point.
- Particles move faster -Temperature increase as heating continues.
Gas Particles have high energy, move quickly.
Vaperization: KE down, particles are getting closer together.
Start to form intermolecular bonds
condensation begins
liquid satrts to form
Freezing; particles arranged in an ofdered manner. Freezing point.
Solid.
Liquid to solid: Freezing
Solid to liquid: Melting
Solid to gas: Sublimation
Gas to solid: Deposition
Liquid to gas: Vaperization
Gas to liquid: Condensation


No comments:
Post a Comment