Brief summary through definition and some new vocabulary:
- Energy level: the amount of energy that electron can possess.
- Ground State: it is when all the electrons are in lowest energy level.
- Excited State: when electrons are in the energy level that different from the lowest level
- Orbital: is the region, like the milky way surrounding around the nucleus that electron occupies in particular energy level
- A shell: is the set of all the orbitals have same energy levels
- Sub-shell: is the set of orbital of the same types (s,p,d,f)
- Electron Configuration: is the notation that describes the orbital in which electron occupies and the total number of electron each orbital
Actually, i find the first chart easier to count and do exercise but its up to you. Here's how ->
Usually in test you will be given question like this: Given element Na, write the electron configuration. Don't freak out yet, follow these steps !
- Look into your periodic table to find that element. In this case, we found Na located in Group IA, period 3
- Then find the element's proton's number, usually in the top left in the box
- We all known that if the element is not ion, the number of electron = number of proton
- So we will be able to know how many electron are there in the element. Okay keep in mind that number.
- then we follow the route on the periodic table from left to write, top to bottom
- So finally we just follow that route till we reach that element
- I can write without think of anything: 1s2 2s2 2p6 3s1
- Follow steps 1 above
- Look backward to the previous row, then find the noble gas ON THE BACK ON YOUR ELEMENT, NOT IN FRONT OF
- Write that noble gas in bracket in this case [Ne]
- Then continue to write the electron configuration till you reach your element. => [Ne] 3s1
Here are some scientist contributed to this concept

- The Pauli Exclusion Principle: in every given energy level, each orbit can only contains 2 electrons
- The Niel Bohr Principle: electron exists in a specific energy states and can be filled up from low level to high level
Valance Electron:
It's basically the number of electron in the outer most shell. Or orbital
Ex: The Valance Electron of Na ( [Ne] 3s1 ) is 1 cause there's only 1 electron in the outer most shell

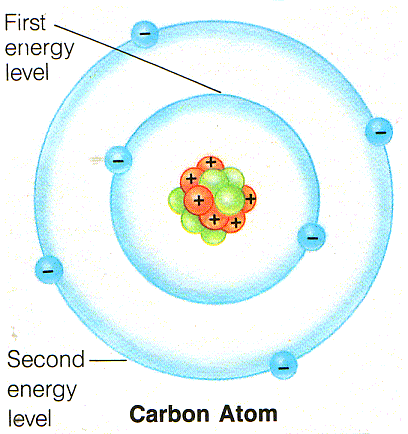






No comments:
Post a Comment