- Synthesis
- Decomposition
- Single Replacement
- Double Replacement
- Combustion
- Neutralization
- SYNTHESIS:
A+B --> AB
Ex: 2 Na + 1 Cl2 -->2 NaCl ( don't forget your balance man )
- DECOMPOSITION:
AB --> A + B
Ex: CaCO3 --> CaO + CO2
- SINGLE REPLACEMENT:
A + BC --> AC + B or
A + BC --> BA + C
Ex: 2 Na + BaCl2 --> 2 NaCl + Ba
- DOUBLE REPLACEMENT:
AB + CD --> AD + CB
WARNING!!: Unless there's a solid formed, the reaction is not occur. And you must write a Net Ionic Equation if it occurs including the states.
Ex: 2 Na3PO4 + 3 Ca(NO3)2 --> 6 NaNO3 + Ca3(PO4)2
Net Ionic Equation: 3Ca (s) + 2 (PO4) (aq) --> Ca3(PO4)2 (s)
- COMBUSTION:
AB + O2 --> AO + BO
Ex: C3H8 + 5O2 = 3CO2 + 4H2O
- NEUTRALIZATION:
Acid + Base --> Salt + H2O
Ex: HCl + NaOH --> NaCl + H2O
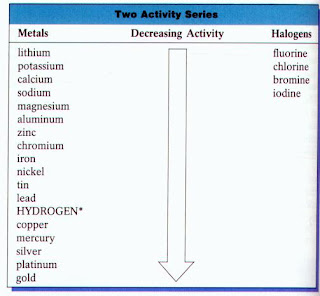
Activity series chart
Solubilites Chart

No comments:
Post a Comment